SORPTION PURIFICATION OF POLLUTANTS IN THE FORM OF MICROORGANISMS AND THEIR NUTRIENT MEDIA IN A SOLITARY PORE SYSTEM OF A CARBON SORBENT
SORPTION PURIFICATION OF POLLUTANTS IN THE FORM OF MICROORGANISMS AND THEIR NUTRIENT MEDIA IN A SOLITARY PORE SYSTEM OF A CARBON SORBENT
Abstract
The article considers the sorption of pollutants on a carbon sorbent. The study of recycled and purified water samples was carried out using photometric equipment. By modeling, it was found that pollutants (in the form of microorganisms and their nutrient media) are mainly immobilized in micropores and, in the future, their study was carried out using electronic optics. With the help of bioinformatic interpretation, sorption interaction of microorganisms subdivided by size factor in a solitary pore system consisting of three types of subsystems: macro-, meso- and micropores is modelled. For a theoretical description of a pore system, an oriented graph is constructed using network theory. Then, using the Roy-Warshall algorithm in a matrix representation, the problem of its optimal routing is solved, with the calculation of a path element for an example. In the future, considering sorption as a single process of transportation and purification of pollutants, the phenomenon of their slip, which inevitably occurs in these cases, was predicted. The determination of the probability of the passage of pollutants using the Bayes theorem, often used in bioinformatics, made it possible to predict, in general, such an equilibrium phenomenon as desorption.
1. Introduction
Recycled (waste) water as a nutrient medium for numerous microorganisms (blue-green algae, protozoa, bacteria and viruses) it causes many harmful phenomena, such as turbidity and unpleasant odor, corrosion of concrete and metals, even such as stainless steels, and also, due to the transfer of a number of serious diseases, poses a real threat to human health. Municipal wastewater containing an increased concentration of iron oxides and phosphates, in general, are excessive nutrient media for the development of algae and protozoa, as well as bacteria and viruses. Thus, due to the widespread use in everyday life of synthetic detergents containing phosphates in high concentrations, the growth and reproduction of blue-green algae occurs in wastewater, causing its active flowering, accompanied by a drop in dissolved oxygen in the water . When the dissolved oxygen content decreases to critical values, many aquatic inhabitants (for example, fish) begin to die, so flowering can lead to the formation of overseas zones. One of the most effective methods of wastewater treatment from such pollutants is the use of carbon sorbents. Close attention is paid to the study of the nature of the adsorption interaction, porosity, as well as the specific surface area of sorbents, since the strengths of the use of these substances are their high absorbing and brightening ability, repeated use, as well as availability and cheapness .
2. Experiment and methods
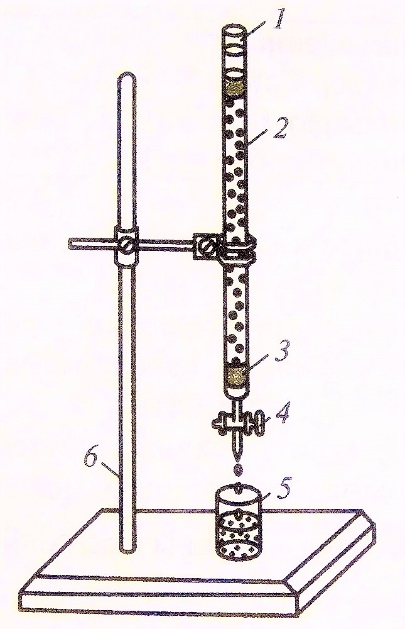
Figure 1 - Filtration column with carbon sorbent
Note: 1 – glass burette; 2 – sorbent layer; 3 – glass wool or fiberglass layer;
4 – a tap to adjust the filtration rate; 5 – a glass for collecting filtrate;
6 – metal tripod with foot
The essence of the method for determining phosphates was to adapt the standard method of "Water and wastewater Research", 20th edition, ascorbic acid method . During the analysis, as a result of the reaction of phosphates and reagent, the measured liquid (wastewater and purified water) was colored blue, the intensity of which was recorded by a highly sensitive silicon photodetector. The radiation source was a point LED with a wavelength of 525 nm. Wastewater according to the results of a series of tests showed an overestimated phosphate content, averaging 5.0 mg/l (ppm), which is explained by the widespread use of various synthetic detergents based on phosphate salts by the population as highly effective water softening reagents. During the filtration of wastewater on the sorbent, in the sorption column, the phosphate content was reduced by almost an order of magnitude, to a value of 0.55 mg/l (ppm).
The essence of the method for determining total iron was the use of EPA-phenanthroline, also known as method 315B . During the analysis, as a result of the reaction of common iron and reagent, the measured sample (wastewater and purified water) was colored orange, the intensity of which was recorded by a highly sensitive silicon photodetector. The radiation source also served as a point LED with a wavelength of 535 nm. Wastewater according to the results of a series of tests showed a total iron content averaging 1.54 mg/l (ppm). During the filtration of wastewater on the sorbent, in the sorption column, the total iron content was reduced by more than an order of magnitude, to a value of 0.14 mg/l (ppm).
Thus, the pore system of the carbon sorbent, effectively retaining various microorganisms in itself, makes the water safer biologically, and also works as an effective means of absorbing phosphates and total iron, which, in turn, are nutrient media for the origin and development of foci of secondary bioorganic pollution of recycled and wastewater.
3. Calculation algorithm and discussion of the results
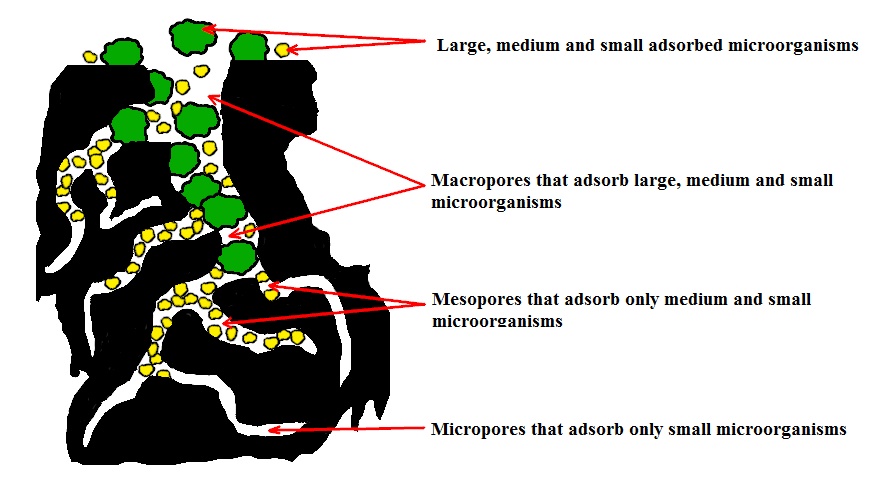
Figure 2 - Model of a solitary pore system
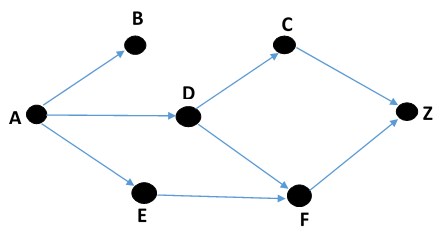
Figure 3 - Digraph describing a solitary pore system
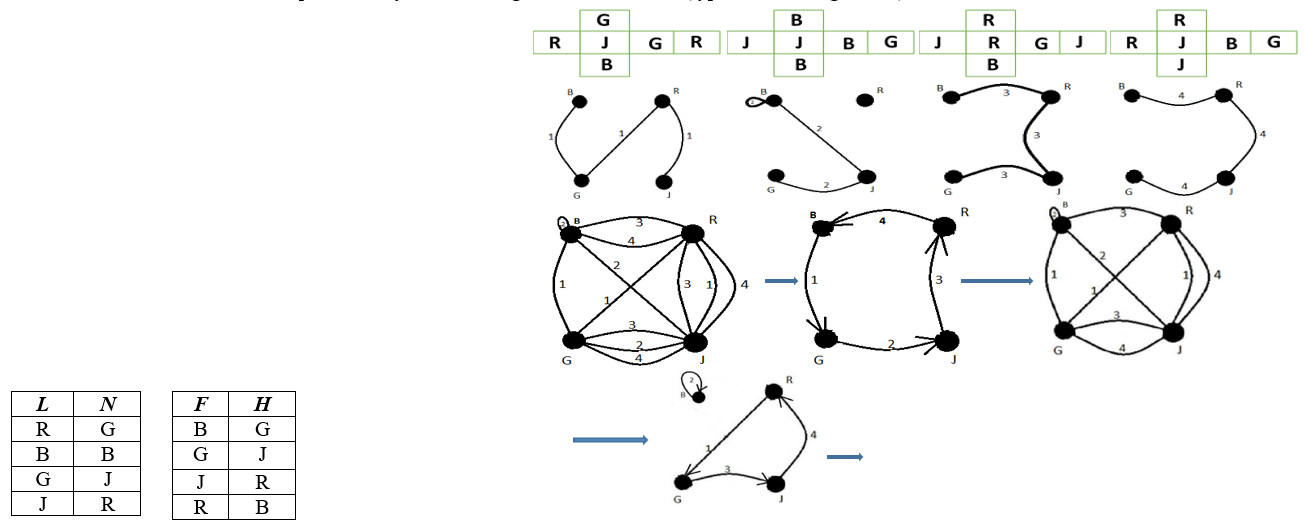
Figure 4 - The cubes

Figure 5 - The adjacency matrix describing the digraph characterizing the pore system

Figure 6 - Case 1
So, there is a path of length 2 from vertex V1 to vertex V3.

Figure 7 - The form of the Roy-Warshall algorithm for manual counting
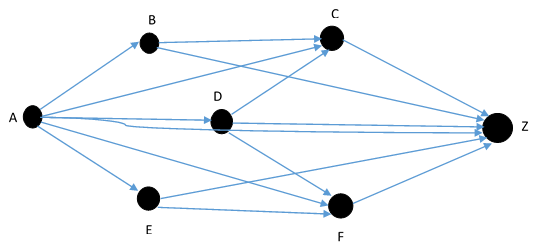
Figure 8 - Deterministic digraph based on transitive closure

Figure 9 - Electronic photo of microorganisms localized in micropores of the pore system (micropore section, large and small bacteria + viral particles)
Then, by Bayes' theorem, we have:
– the probability that pollutants will make a slip from macropores;
– the probability that pollutants will make a slip from mesopores;
– the probability that pollutants will make a slip from micropores.
Then, the total probability will be equal to the sum:
That's why sorbents for wastewater treatment and discoloration tend to be manufactured with highly developed meso- and microporosity. Macroporous sorbents, which do not provide significant resistance to the flow, are produced mainly for cleaning gases from dust and particles suspended in them.
4. Conclusion
The use of carbon sorbent makes it possible to achieve high results in the treatment of wastewater and recycled water from various pollutants represented by various microorganisms and their nutrient media, which makes it possible to reuse purified and discolored water in everyday life and the national economy. Sorbents based on carbon materials are highly efficient and environmentally friendly systems for disinfection and demineralization of wastewater. The consequence of their use is a successful fight against microorganisms and their nutrient media, increasing the level of environmental safety to the maximum possible. The sorbent itself is not an expensive substance and, if necessary, can be easily replaced and regenerated.
