АНТИОКСИДАНТЫ ПОВЫШАЮТ УСТОЙЧИВОСТЬ ПРОРОСТКОВ ГОРОХА К ДЕФИЦИТУ ВОДЫ
АНТИОКСИДАНТЫ ПОВЫШАЮТ УСТОЙЧИВОСТЬ ПРОРОСТКОВ ГОРОХА К ДЕФИЦИТУ ВОДЫ
Аннотация
Введение: Митохондрии, являясь «энергетическими станциями» клетки, в условиях стресса повышают генерацию активных форм кислорода (АФК). Избыток этих интермедиантов приводит к активации перекисного окисления мембранных липидов и набуханию митохондрий. Что может сопровождаться выходом апоптогенных белков в цитоплазму и последующей инициацией апоптоза. Предполагается, что антиоксиданты, подавляющие избыточное образование АФК митохондриями, могут повышать резистентность организма к стрессовым факторам. В связи с этим целью данной работы было изучение возможности использования антиоксидантов (амбиола (2-метил-4-диметиламинометил-бензилимидазол-5-ол-дигидрохлорида)); Карнитинат n-2-этил-6-метил-3-гидрокси-пиридин и ресвератрола (3,5,4'-тригидрокси-транс-стильбена) в качестве адаптогенов к стрессовым воздействиям, в частности к дефициту воды.
Методы: Функциональное состояние митохондрий определяли по: исследованию уровня перекисного окисления липидов с помощью спектрофлуориметрии, анализу жирнокислотного состава мембран митохондрий методом хроматографии и по определению скорости дыхания митохондрий методом полярографии.
Результаты: Дефицит воды приводил к активации ПОЛ, что вызывало снижение содержания С18 и С20 жирных кислот (ЖК) в мембранах этих органелл. Содержание линоленовой и линолевой кислот уменьшалось на 12,46% и 21% соответственно. При этом индекс двойных связей С20 ЖК снижался с 0,120±0,001 до 0,069±0,001. Изменения физико-химических свойств мембран митохондрий сопровождались 40% снижением максимальных скоростей окисления НАД-зависимых субстратов и 30% снижением эффективности окислительного фосфорилирования. Обработка семян гороха 10–9 М растворами исследуемых антиоксидантов предотвращала активацию перекисного окисления липидов в мембранах митохондрий, предупреждала пероксидацию С18 и С20 ЖК и изменение биоэнергетических характеристик митохондрий, обусловленное стрессом.
Выводы: Предполагается, что предотвращение пероксидации С18 жирных кислот свидетельствует о поддержании антиоксидантами содержания кардиолипина в мембранах митохондрий на исходном уровне. Это обеспечивало эффективное функционирование дыхательной цепи митохондрий и, следовательно, сохранение энергетического обмена на высоком уровне. Что способствовало повышению устойчивости растений к стрессу.
1. Introduction
Water is the dominant component of plants, accounting for up to 95% of their total mass. It is critically important throughout the entire life cycle. Water maintains turgor, transports nutrients, and participates in transpiration and thermoregulation. An optimal water regime creates favorable conditions for biochemical reactions in the plant organism, ensuring high plant productivity. Insufficient or excessive soil moisture negatively affects a number of physiological and biochemical processes in the plant. With water deficiency, the content of free water in cells decreases first. This alters the structure of the hydration shells of cytoplasmic proteins, which negatively affects the functioning of enzymes . Under conditions of water stress, cell division processes and, especially, cell elongation slow down, which is accompanied by the formation of small cells. During severe drought, the biosynthesis of organic substances slows and hydrolysis increases, which disrupts normal plant growth . Even after a short-term severe drought, plants do not return to normal metabolism . Conversely, excessive watering leads plants to a state of anaerobiosis. When the soil is excessively moistened, plants are in a state of anaerobiosis, which hinders the flow of water and minerals into plant roots, increases respiration and simultaneously reduces the activity of the process of organic matter synthesis, and disrupts the water regime of plants .
The distribution of plants and animals is fundamentally limited by water availability, influencing the species found in different ecosystems. Excessive soil moisture leads to a lack of oxygen (anaerobiosis) for plants. This state negatively impacts their ability to draw in water and minerals through their roots, increases their energy expenditure through respiration, and slows down the production of essential organic substances, thereby compromising their overall water management . Therefore, the presence of water is a crucial limiting factor for the distribution of life in ecosystems, with the quantity of water directly influencing the types of flora and fauna found in a region.
Adaptation to adverse environmental conditions, such as water scarcity, is known to require significant energy expenditure. Energy metabolism, and particularly mitochondrial function, plays a key role in the body's response to stress. Therefore, the focus of this study was on mitochondria. Within cells, mitochondria are the leading generators of ROS. Normally, only a small portion (1–3%) of the oxygen used by mitochondria is converted into reactive oxygen species (ROS), which, in turn, act as signalling molecules, participating in the regulation of plant growth and development. Steady-state ROS levels in tissues are maintained by enzymatic and non-enzymatic systems that control their formation or breakdown. However, in unfavorable conditions, this balance is disrupted, leading to excessive generation of ROS, which, depending on the severity of the stress factors, can either stimulate adaptation processes or cause disturbances in metabolism of cells. ROS, interacting with lipids of mitochondrial membrane, initiate LPO, leading to mitochondrial swelling and the initiation of apoptosis . Based on this, it can be assumed that antioxidants that reduce the excessive production of reactive oxygen species (ROS) by mitochondria may increase the body's ability to withstand stress, which indicates the possibility of their use as plant growth regulators.
We selected preparations from various classes of chemical compounds as objects of study: a 5-hydroxybenzimidazole derivative — ambiol: (2-methyl-4-dimethylaminomethyl-benzylimidazole-5-ol-dihydrochloride) (AMB); a 3-hydroxypyridine derivative — Carnitinate n-2-ethyl-6-methyl-3-hydroxy-pyridine (CAR) and an innate antioxidant — resveratrol (3,5,4'-trihydroxy-trans-stilbene) (RV):

Figure 1 - Antioxidants
Note: Ambiol; Carnitinate N-2-ethyl-6-methyl-3-hydroxy-pyridine; Resveratrol
2. Research methods and principles
The experimental material consisted of mitochondria obtained from 5-day-old etiolated seedlings of the Pisum sativum L. Flora 2 pea cultivar.
2.1. Germination of pea seeds
The seeds were first cleaned using a soapy solution and then treated with a 0.01% potassium permanganate solution. The control group seeds were soaked in distilled water for one hour, while the experimental group seeds were soaked in a solution of the studied antioxidants of the appropriate concentration.
2.2. Optimizing antioxidant concentrations during seed treatment
Experiments showed that treating pea seeds with antioxidants at a concentration of 10-9M promoted faster germination and seedling growth. Specifically, hypocotyl weight increased by 35%, and root weight by 20%. Using solutions with other antioxidant concentrations did not produce such pronounced effects.
2.3. Investigational drugs
Ambiol (AMB), Carnitinate n-2-ethyl-6-methyl-3-hydroxy-pyridine (CAR) were first synthesized at Emanuel Institute of Biochemical Physics of Russian Academy of Sciences. Registration number (certificate) of Ambinol trademark: 258534. Registration number (certificate) of Carnitinate n-2-ethyl-6-methyl-3-hydroxy-pyridine — RU2817094C1. Ambiol and Carnitinate n-2-ethyl-3-methyl-3-hydroxy-pyridin were synthesized by the Institute in January 2024. Resveratrol — used by Sigma-Aldrich, USA.
2.4. Mitochondria isolation from the epicotyls of etiolated seedlings
Mitochondria were extracted from etiolated pea seedlings, measuring 3 to 6 centimeters in length, employing a differential centrifugation technique as described in reference . First, seedlings epicotyls of the crushed in a buffer solution consisting of 0.4 M sucrose, 5 mM EDTA, 20 mM KH2PO4 (pH 8.0), 10 mM KCl, 2 mM dithiothreitol and 0.1% BSA free of fatty acids (FA). The resulting homogenate was centrifuged at 25,000 g for 5 minutes. Next, the precipitate was re-suspended in 8 ml of a rinsing medium (0.4 M sucrose, 20 mM KH2PO4 (pH 7.4)), 5 mM EDTA, 10 mM KCl and 0.2% BSA (free from FA) and centrifuged at 3000 g for 3 minutes. The filler liquid was centrifuged at 11,000 g for 10 min, precipitating the mitochondria. The precipitate was re-suspended in 2-3 ml of medium (0.4 M sucrose, 20 mM KH2PO4 (pH 7.4)), 0.1% BSA (free from FA) and re-centrifuged under the identical conditions.
2.5. Measuring mitochondrial oxygen consumption rates
Mitochondrial oxygen consumption rates determined by the polarographic method on an LP-7 polarograph (Czech) with a Clark electrode. Incubation medium included 0.4 M sucrose, 20 mM HEPES-Tris buffer (pH 7.2), 5 mM KH2PO4, 4 mM MgCl2 and 0.1% BSA.
2.6 Lipid Oxidation (LPO) Activity
Lipid peroxidation levels were determined using a fluorescence-based assay . Mitochondria, containing 3 to 5 milligrams of protein, were subjected to lipid extraction using a solvent composed of chloroform and methanol in a 2:1 volume ratio. The mitochondria were mixed with the chloroform-methanol solution at a ratio of 1:10 (volume to volume). Fluorescence was recorded in 10-mm quartz cuvettes on a FluoroMax-HoribaYvonGmbH spectrofluorimeter (Germany). Fluorescence was measured at an excitation wavelength of 360 nm and an emission range of 420-470 nm. The data were quantified as arbitrary fluorescence units per milligram of protein.
2.7. Methyl esters of fatty acids (FAMEs)
To prepare mitochondrial membrane lipids for analysis, they underwent acid methanolysis to yield fatty acid methyl esters (FAMEs) , . The FAMEs were then isolated by hexane extraction, and the resulting solutions were subjected to analysis.
2.8. Quantitative assessment FAMEs
For quantitative determination of the FAME composition, a Crystal 2000M chromatograph (Russia) with a flame ionization detector and a DB-1 quartz capillary column was used. FAMEs were identified by comparing their retention times to existing research data . The proportion of each FAME within a sample was then calculated by dividing the area under its peak on the chromatogram by the total area of all identified FAME peaks. The peak areas from three measurements varied by a maximum of 5% (based on standard deviation).
2.9. FAMEs identification
FAMEs in the samples were identified using mass spectrometry, with data acquired after chromatographic separation on a Hewlett Packard-6890 (USA) under gas chromatography-like conditions. The mass spectra were recorded via electron impact ionization (70 eV) at a scanning speed of 1 second per decade of mass, from 40 to 400 Daltons.
2.10. Model of mitochondrial "aging"
Mitochondria (2–3 mg protein) were placed in 0.5 ml of a medium consisting of 100 mM potassium chloride, 10 mM HEPES buffer, 1 mM potassium dihydrogen phosphate, with a pH of 7.4. Mitochondria were subjected to a 20–25 minute incubation at room temperature.
2.11. Statistical processing
Experimental data underwent statistical analysis, which involved calculating the average values and their associated standard deviations. Differences between groups were considered statistically significant if the probability value (P) was less than or equal to 0.05.
2.12. The experiment was carried out using the following reagents:
Sucrose, Tris, EDTA, FCCP, ADP, BSA (free from FA), malate, glutamate (Sigma Aldrich, USA); HEPES (“MP Biomedicals,” Germany), methanol, chloroform (Merck, Germany)The work was carried out on mitochondria of 5-day-old etiolated pea seedlings (Pisum sativum L.) Flora 2 cultivar.
3. Results and discussions
To simulate stress effects, we utilized a mitochondrial “aging” model. This model allows for the identification of drug concentrations that lower mitochondrial ROS generation, thereby determining the antioxidant concentrations needed to block lipid peroxidation activation. The activation of lipid peroxidation was tracked via the fluorescence of its end products, Schiff bases . We observed that “aging” in pea seedling mitochondria led to increased lipid peroxidation, evidenced by a nearly threefold rise in Schiff base fluorescence in mitochondrial membranes (Fig. 2). The addition of drugs to the mitochondrial incubation medium reduced lipid peroxidation intensity, with the effect being dependent on drug concentration.
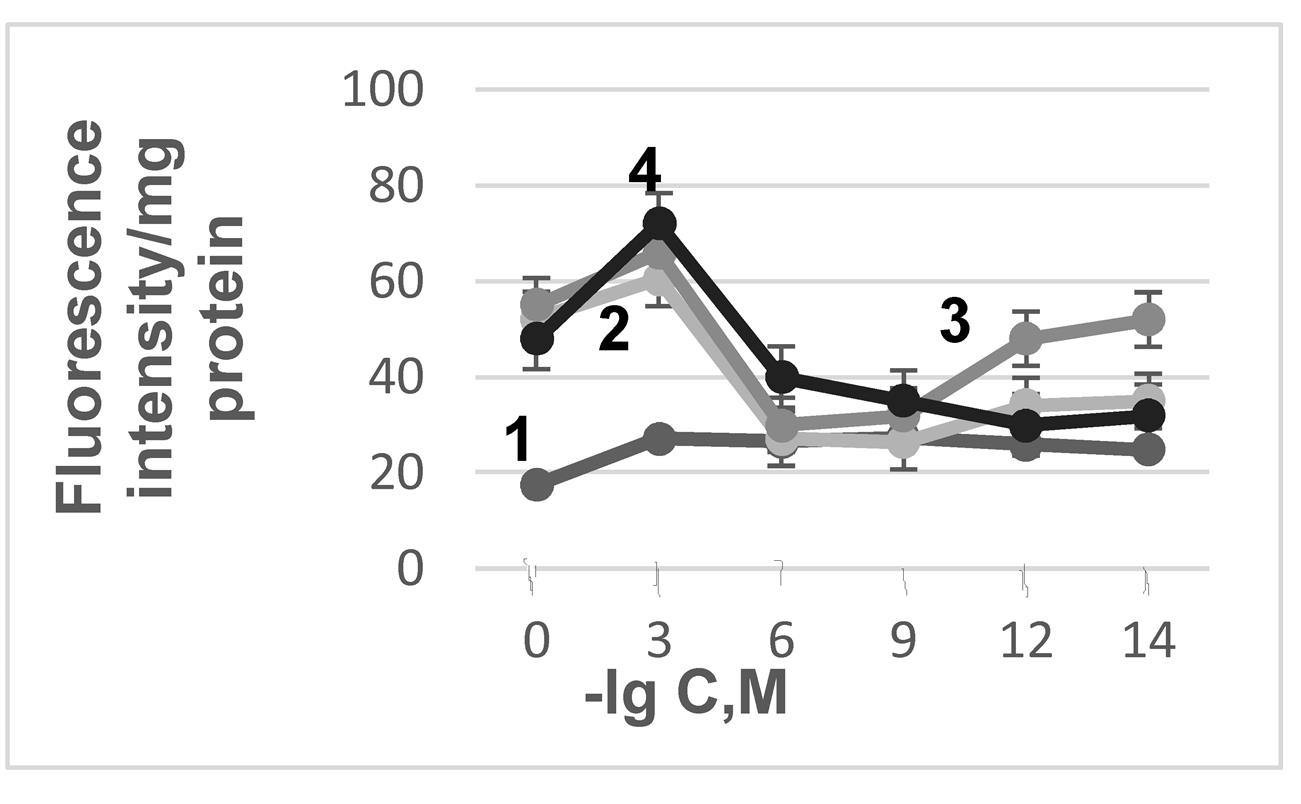
Figure 2 - The relationship between antioxidant (AO) concentration, mitochondrial "aging" and the fluorescence intensity of LPO products
Note: 1 – control; 2 – "aging"+CAR; 3 – "aging"+AMB; 4 – "aging"+RES
Under water-stressed conditions, plant membranes showed modified 18-carbon fatty acid profiles.

Figure 3 - Fluorescence spectra of LPO products in mitochondrial membranes from 5-day-old etiolated pea seedlings under water deficit (WD) and after antioxidant seed treatment in conditions of water shortage
Note: 1 – WD; 2 – WD+RES; 3 – WD+AMB; 4 – WD+CAR; 5 – control
Table 1 - Effects of water shortage (WD), Carnitinate N-2-Ethyl-6-methyl-3-hydroxypyridine (CAR), Ambiol (AMB), Resveratrol (RSV) on the FA content in the pea seedlings mitochondrial membrane
Fatty Acids | Control | Water shortage | Water shortage + CAR-3-HP | Water shortage +AMB | Water shortage +RSV |
Relative percentages | |||||
12:0 | 0.37±0.03 | 0.96±0.01 | 0.40±0.03 | 0.50±0.02 | 0.56±0.02 |
14:0 | 0.66±0.01 | 1.58±0.02 | 0.67±0.02 | 0.69±0.02 | 0.61±0.03 |
16:1ω7 | 0.94±0.03 | 1.9±0.04 | 0.93±0.03 | 1.00±0.01 | 1.66±0.05 |
16:0 | 18.0±0.75 | 22.40±0.03 | 18.87±0.13 | 18.0±0.50 | 14.69±0.10 |
17:0 | 0.99±0.05 | 0.9±0.12 | 1.00±0.01 | 1.0±0.16 | 0.42±0.11 |
18:2 ω6 | 50.0±0.08 | 43.77±0.40 | 50.00±0.05 | 50.97±0.06 | 50.74±0.09 |
18:3 ω3 | 13.2±0.02 | 10.4±0.01 | 12.53±0.03 | 12.74±0.09 | 12.34±0.04 |
18:1 ω9 | 2.78±0.40 | 3.49±0.37 | 2.67±0.20 | 3.76±0.12 | 3.05±0.10 |
18:1 ω7 | 0.67±0.10 | 0.64±0.24 | 0.61±0.10 | 0.53±0.03 | 1.00±0.26 |
18:0 | 3.0±0.18 | 5.83±0.32 | 2.48±0.12 | 3.00±0.12 | 3.81±0.15 |
20:3 ω6 | 1.17±0.01 | 0.5±0.01 | 1.17±0.02 | 1.38±0.01 | 1.3±0.04 |
20:2 ω6 | 2.46±0.01 | 1.48±0.01 | 3.69±0.05 | 3.02±0.03 | 3.5±0.02 |
20:1 ω 9 | 2.29±0.01 | 1.54±0.01 | 2.41±0.01 | 1.56±0.01 | 2.57±0.01 |
20:1 ω7 | 1.26±0.03 | 1.10±0.01 | 1.25± 0.01 | 1.0±0.02 | 1.50±0.01 |
20:0 | 1.36±0.03 | 1.9±0.03 | 0.51±0.02 | 1.00±0.05 | 0.97±0.03 |
22:0 | 0.85±0.01 | 1,61±0.03 | 0.81±0.02 | 0.85±0.01 | 1,00±0.02 |
Under water-stressed conditions, plant membranes showed modified 18-carbon fatty acid profiles . This involved a decline in C18 fatty acids like linoleic and linolenic, alongside an increase in stearic acid , . Comparable shifts were also detected in C20 fatty acids. A shift was observed, with a reduction in the amount of unsaturated fatty acids and a corresponding rise in saturated fatty acids. This change was quantified by a drop in the double bond index, from 0.120±0.001 to 0.069±0.001, and a decrease in the unsaturation coefficient for 20-carbon fatty acids, from 5.08±0.03 to 3.00±0.16. It's important to note that Very long chain fatty acids (VLCFAs), characterized by their length of more than 18 carbon atoms, are known to be essential for plant life, fulfilling both physiological and structural functions. Their involvement in the organization of membrane domains is also a possibility . According to A.V. Zhukov and M. Shumskaya Zhukov , the extended structure of very long chain fatty acids (VLCFA) is key to their role in cell membranes. Their length enables them to span both leaflets of the lipid bilayer. This unique characteristic, the author proposes, contributes to membrane stability, particularly under stressful conditions. Furthermore, it is hypothesized that unsaturated fatty acids containing 20 carbon atoms (C20-FA) within the mitochondrial membranes of seedlings may be responsible for the seedlings' ability to withstand water scarcity.
Modifications to mitochondrial membrane physicochemical properties impacted the activity of respiratory chain enzymes. NAD-dependent substrate oxidation rates diminished by 40%, and oxidative phosphorylation efficiency (V3/V4) decreased by 30% (Table 2). Succinate oxidation was more tolerant of water stress, with only a 10–15% decline. The addition of 10 µM vitamin K3 (menadione) to the the mitochondrial incubation medium aided in restoring electron transport rates at the respiratory chain's beginning, suggesting complex I inhibition.
Table 2 - The rates of NAD-dependent substrate oxidation by pea seedling mitochondria, as affected by water deficiency and the tested antioxidants
Group | V2 | V3 | V4 | V3/V4 | FCCP |
Control | 22.90±1.20 | 71.40±1.40 | 31.32±1.10 | 2.28±0.01 | 72.80±1.80 |
WD | 15.00±1.10 | 42.84±2.00 | 26.60±1.00 | 1.61±0.02 | 41.10±2.00 |
WD+AMB | 24.74±1.40 | 76.50±1.20 | 33.00±1.25 | 2.32± 0.01 | 77.40±2.10 |
WD+CAR | 25.14±1.21 | 80.45±1.90 | 32.19±1.52 | 2.50±0.01 | 74.65±1.75 |
WD+RES | 18.76±1.00 | 69.60±1.31 | 31.64±1.40 | 2.20±0.02 | 70.22±2.00 |
Note: rates: ng O2 atom/mg protein × min (n=10). Incubation medium: 0.4 M sucrose, 20 mM HEPES-Tris buffer (pH 7.2), 5 mM KH2PO4, 4 mM MgCl2, 10 mM malate, 10 mM glutamate. Additional additives: 200 μM ADP, 10-6 M FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone). Legend: V2 – rates of substrate oxidation, V3 – rates of substrate oxidation in the presence of ADP; V4 – oxidation rate under resting conditions (rates of substrate oxidation when ADP is exhausted)
4. Discussion
The decline in mitochondrial complex I activity during drought conditions is likely linked to the peroxidation of unsaturated fatty acids, particularly linoleic and linolenic acids, which are major components of cardiolipin . This peroxidation may reduce cardiolipin levels in the inner mitochondrial membrane, consequently diminishing mitochondrial functional capacity .
The functional consequences for mitochondria were also reflected in observable physiological changes, specifically seedling growth. Water scarcity suppressed seedling growth (Fig. 4), a result consistent with findings in the literature , .
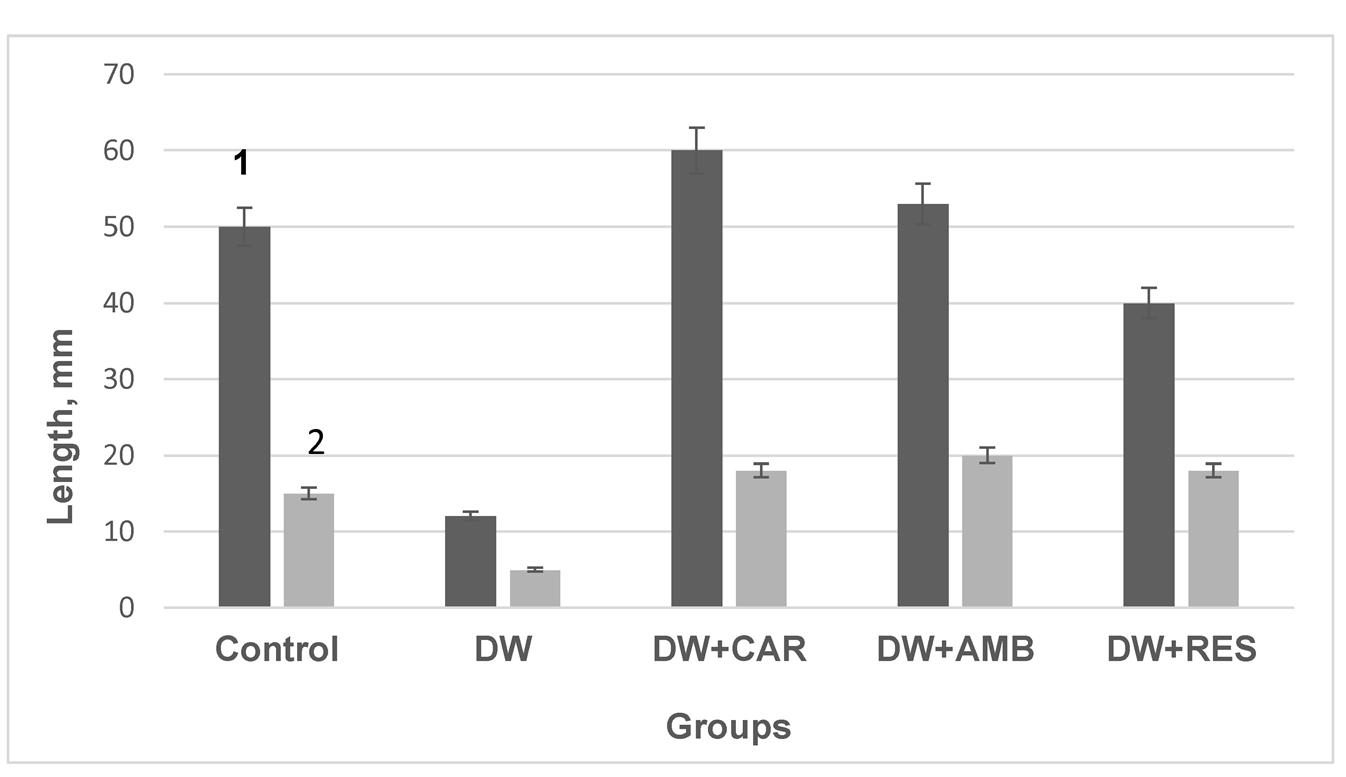
Figure 4 - Effect of water scarcity, CAR, AMB, and RES on pea seedling shoot (1) and root (2) growth
5. Conclusion
This study demonstrates that the investigated antioxidants possess anti-stress effects. The reduction in lipid peroxidation (LPO) suggests these drugs suppress free radical oxidation reactions. Specifically, by protecting phospholipids, especially cardiolipin, the antioxidants likely help maintain the structure and function of the mitochondrial respiratory chain . This promotes the efficient operation of mitochondrial electron transport chains, thereby enhancing the plants' ability to withstand stress. Thus, CAR, AMB, RES can be used as a plant growth regulator in concentrations at which they exhibit antioxidant activity.
